Printed Spinal Implant Receives FDA Approval
August 12, 2016
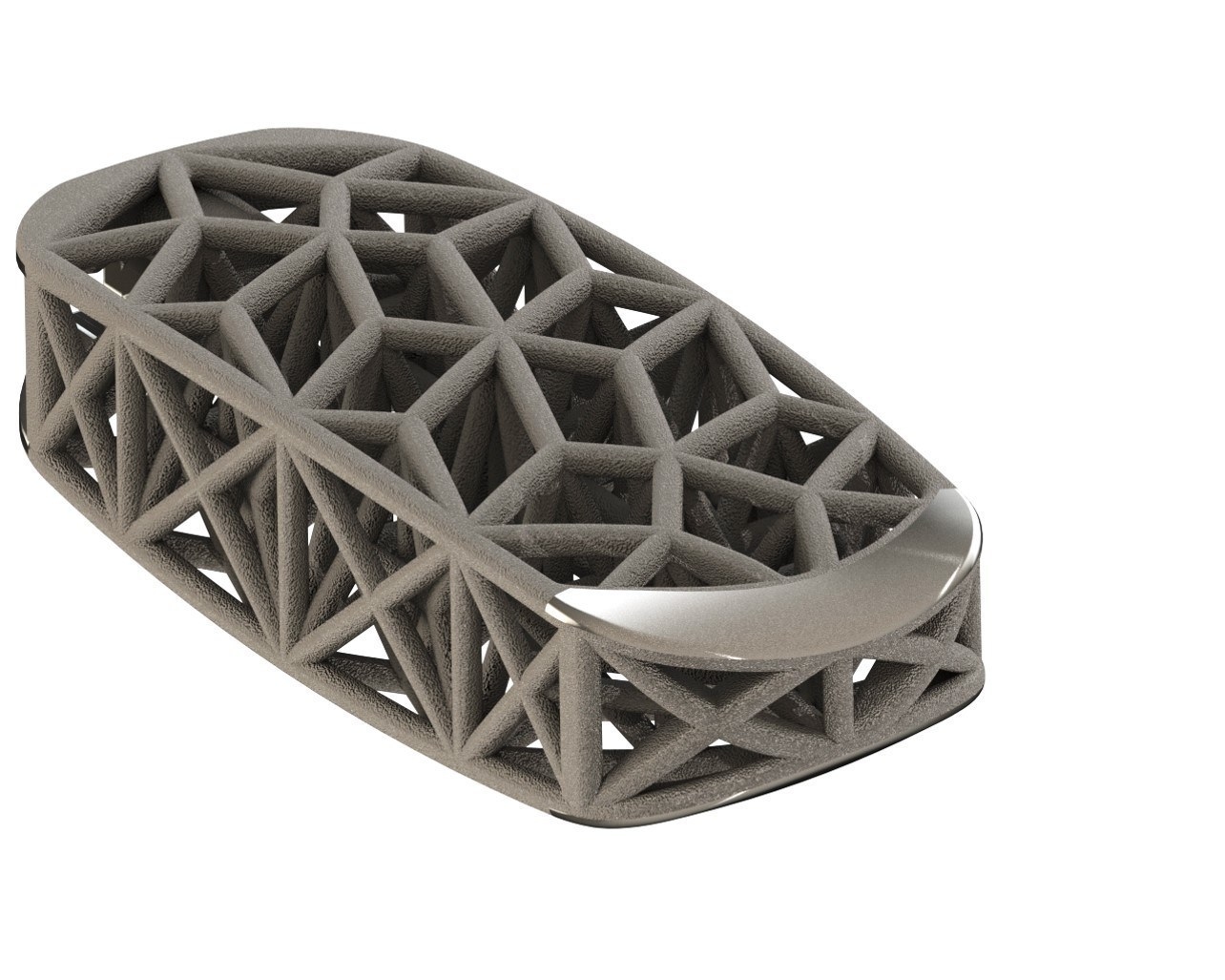
The FDA has given 510K clearance to 4WEB Medical for its 3D-printed Lateral Spine Truss System, a titanium interbody fusion device used to repair spinal injuries.
“4WEB has achieved a significant milestone that allows us to address the majority of fusion procedures and approaches performed today. We now offer Truss Implant Technology for every mainstream surgical technique used in spine surgery,” said Jim Bruty, senior vice president, Sales and Marketing of 4WEB. “4WEB will continue to be on the cutting edge with meaningful innovation in spine surgery by pioneering implant function through structural design.”
The truss will include a variety of sizing options and integrated instrumentation, as well as single-sterile packaging to ensure sterility, improve operating room efficiency and ensure access to global markets where such packaging is required.
Interbody spacers and implants are a rapidly growing market that generates billions in revenues. Fusion procedures join together and immobilize injured or damaged sections of the spine. A number of companies have designed similar implants, including Medicrea, Stryker and Joinmax.
“The Lateral Spine Truss System represents a significant advancement in treatment options for lateral spine surgery,” said Frank Cammisa, MD, professor and chief emeritus of the Spine Service at Hospital for Special Surgery in New York City. “As evidenced in recent porous metal product launches, many companies have failed to recognize the full design potential of what 3D printing brings to the orthopedic arena. 4WEB’s proprietary truss implant technology leverages fundamental engineering principles in structural mechanics to address important clinical issues such as implant subsidence after lateral access surgery.” RT-4WEB is named for the geometry the company uses as the building block for its high-strength, lightweight web structures. The company also offers cervical, ALIF, posterior and osteotomy truss systems, and is developing truss implant designs for knee, hip and other orthopedic procedures. The knee implant is being developed via a $1 million grant the company received from the National Science Foundation.
“I believe the future of orthopedic design and fabrication is customized implants,” CEO Jessee Hunt told the Dallas Morning News. “My vision is 20 to 30 years down the road, you’d have a turnkey solution where a patient would come into the hospital, get diagnosed, imaged, an implant design would be created based on that image and the implant would be printed and would meet the patient in the operating room the same day. This really efficient, turnkey operation is the vision of what’s possible. We keep our eye on that ball and drive that direction.”
Source: Dallas Morning News
Subscribe to our FREE magazine, FREE email newsletters or both!
About the Author
Brian Albright is the editorial director of Digital Engineering. Contact him at [email protected].
Follow DE